half life formula for zero order reaction
The half-life of a Zero-th order reaction is t A0 2kHere I derive this from the Integrated Rate LawAsk me questions. A Product The rate law of zero order kinetics is.
Solved Which Of The Following Represents The Equation For A Zero Order Course Hero
The formula for the half-life of different reactions is given below.

. Formula for half life of a zero order reaction is Formula for half life of a zero order reaction is. Transfer function of a system is given by GS b0 a1Sa0. The half-life of a first-order reaction is given as t 12 0693k.
The half-life of a reaction is defined as the time required for the reactant concentration to fall to one half of its initial value. For a general reaction. Sample Questions Ques 1.
The rate constant for the reaction can be determined from the slope of the line which is equal to -k. Order of a system is the maximum power of S in the characteristic equation. We can identify a 0 1 st or 2 nd order reaction from a plot of A versus t by the variation in the time it takes the concentration of a reactant to change by half.
Correct option is A Half life of zero order reaction is given by t 21 2ka where a is initial concentration of reactants. Because this equation has the form y mx b a plot of the concentration of A as a function of time yields a straight line. Half-life of a first.
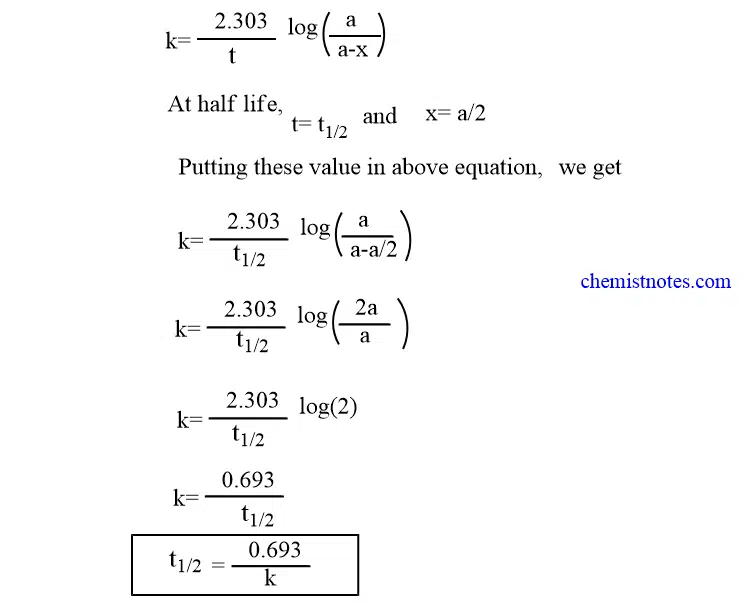
The rate constant for a zero-order reaction is measured in molL -1 s -1. The half-life of a reaction t12. Thus for t t 12 A t ½ A o The integrated rate constant for the zero-order reaction is given by This is an expression of the half-life of a zero-order reaction.
For a zero-order system S will have a power zero and Transfer function will be a constant value. Half-Life of Zero Order Reaction. Zero-Order Half-Life Half-life t½ or half-time is defined as the time period required for the concentration of drug to decrease by one-half.
Half life of Zero order reaction formula is the time at which the initial concentration of reactant becomes half and is represented as T12 C0 2k or Half Life of Zero Order Reaction Initial Concentration for Zero Order Reaction 2Rate Constant of Zero Order Reaction. Report fracC_0K fracC_02K frac2C_0K frac3C_02K Detailed Solution Download Solution PDF For a zero order reaction k fracleftRright_0-leftRrightt. Thus the graph will look like as follows Half-Life versus Initial Concentration graph of zero order reaction.
Previous Half-life 2nd order rxn Current half-life zero order Rxn Next. The Half-Life of Zero Order Reaction calculator computes the half-life in nuclear decay for a zero order reaction. K R 0 R t.
A0 A kt Where A0. I Half-Life of a Zero Order Reaction. When t t½.
We know that the half-life of a zero order reaction is given by t 1 2 A 0 2 k which is again in the form of y mxc where y here will be equal to t 1 2 and x will be equal to A 0 with m as 1 2 k and intercept will be zero. However the half-life of a zero-order reaction increases as the initial concentration increases. Half life of zero order reaction is directly proportional to the initial concentration of reactants.
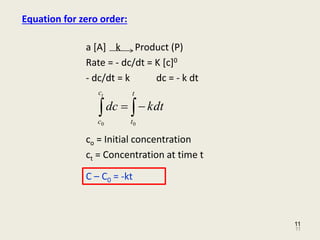
For the reaction given as A B A is reactant and B is a product Rate -dA dt kA0 -dA dt k dA -k dt Now Integrating both sides we get. It is the time in which the concentration of a reactant is reduced to one-half of its initial concentration. If an increase in reactant increases the half life the reaction has zero-order.
The integrated rate law for the zero-order reaction A products is A_t -kt A_0. Was this answer helpful. 0 0 Similar questions.
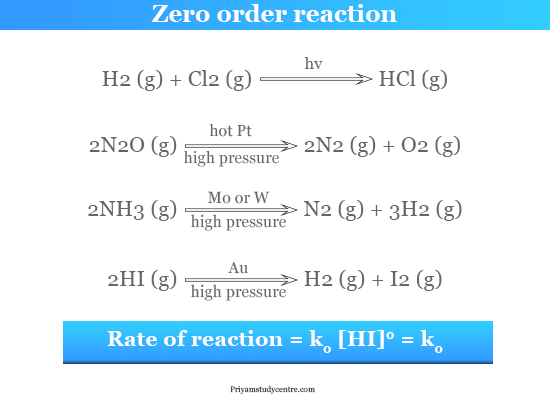
A -kt c Where c constant of integration At time t 0 A A0. In the case of a zero-order reaction the rate of reaction depends on the zeroth power of the concentration of reactants. Half life formula for Zero order reaction A zero order reaction implies that the rate of the reaction does not depend on the concentration of the reactant.
When t t12 R ½ R0. For a zero-order reaction the integrated rate law is. 2 A0kt121 A0 2 A0-1 A0kt12 t121k A0 3 One important thing to notice in the above reaction is this the half-life of a second-order reaction depends only on the initial concentration in contrast to the first-order reactions.
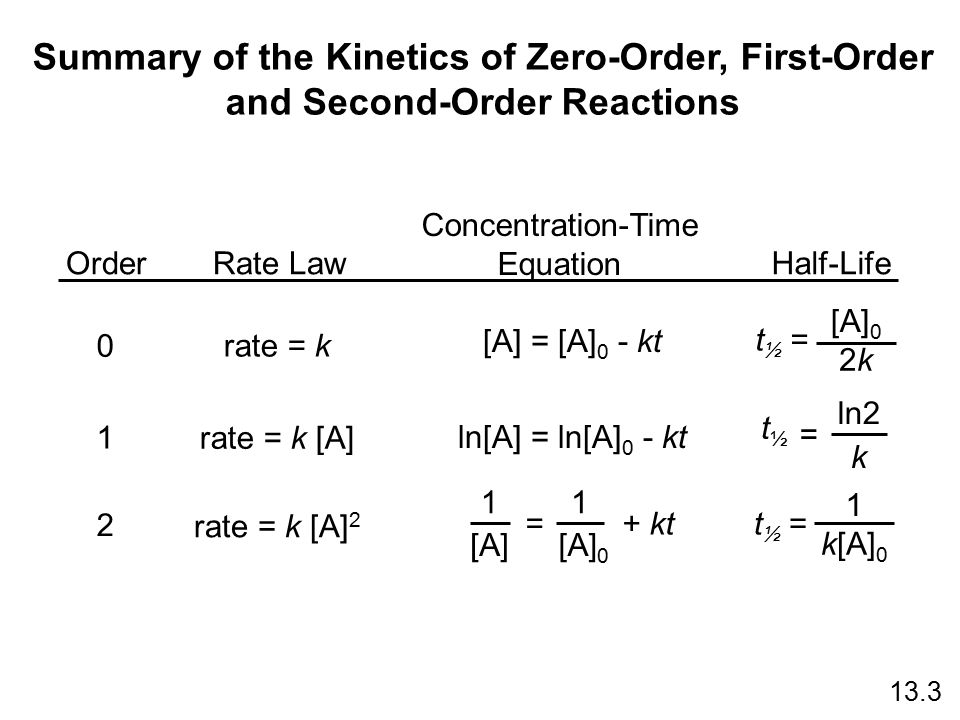
The half-life of a zero-order reaction the formula is given as t12 R02k The half-life of a first-order reaction is given as t12 0693k The half-life of a second-order reaction is given by the formula 1kR0. The half-life of a zero-order reaction the formula is given as t 12 R 0 2k. Equations for both differential and integrated rate laws and the corresponding half-lives for zero- first- and second-order reactions are summarized in the table below.
It is represented by t12. The mathematical expression that can be employed to determine the half-life for a zero-order reaction is t12 R 02k For the first-order reaction the half-life is defined as t12 0693k And for the second-order reaction the formula for the half. The half-life of a second-order reaction is given by the formula 1kR 0.
For a zero order reaction Half life decreases with decreasing concentration For a 1st order reaction Half life is constant.

Zero Order Reactions Video Kinetics Khan Academy
Derive Half Life For Zero Order And First Order Reaction Chemistry Point
Kinetics And Drug Stability Ed

Half Life Expressions Chemistnate

Half Life Of Zero Th 0th Order Reaction Derivation Youtube
First Order Reaction Definition Example Half Life Period Chemist Notes

Half Life Of A Zero Order Reaction Is 250sec T75 T100 Of The Reaction Respectively In Sec Are Edurev Neet Question
Zero Order Reaction Definition Examples Formula
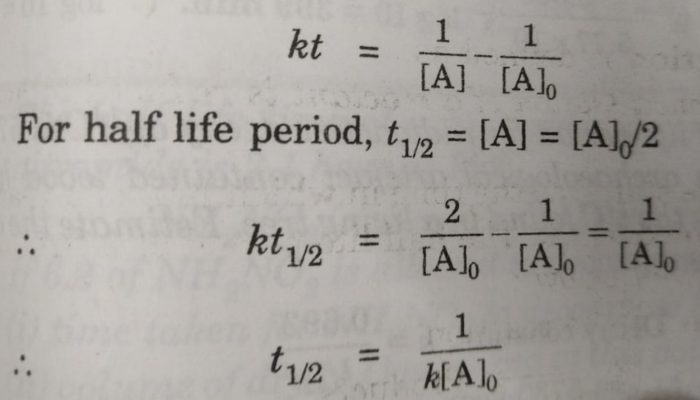
Half Life Period Of A Reaction Chemical Kinetics

Zero Order Reaction Definition Examples Formula
Summary Of The Kinetics Of Zero Order First Order Ppt Download

Kinetics Reaction Rates Mechanisms Ppt Download

Zero Order Reactions Chemistry Class 12 Iit Jee Main Advanced Neet Aipmt Askiitians Youtube
A Derive The General Form Of The Expression For The Half Life Of A First Order Reaction Sarthaks Econnect Largest Online Education Community

Derive The Relationship Between Half Life Period And Rate Constant Of A Zero Order Reaction

Integrated Rate Laws Zero First Second Order Reactions Chemical Kinetics Youtube

Calculate The Half Life Period For Zero Order Reaction 12 Chemical Kinetics Chemistry Youtube
